Hydrogen Cyanide
Hydrogen cyanide (HCN) is the active toxic ingredient in the pesticide Zyklon B, which has been around since the 1920s (see the entry Zyklon B for details on this product). At room temperature, HCN is a colorless liquid, but it boils already at 25.7°C (78.3°F). It dissolves easily in water and reacts readily with iron salts.
HCN has no effect on microbial lifeforms at levels prescribed for disinfestations, hence cannot be used for disinfections. However, it is lethal above certain concentrations to multi-cellular lifeforms, such as insects and mammals. This chemical blocks a cell’s ability to use oxygen for its metabolism, and hence suffocates it on a cellular level. As a result, the blood stays rich in oxygen, becoming over-saturated with it as the poisoning progresses. The visible hallmarks of cyanide poisoning are therefore a distinct pink complexion, and pinkish-red rather than purple-bluish death marks (livor mortis).
Insects – and in particular insect eggs – are considerably less sensitive to hydrogen-cyanide poisoning than are warm-blooded animals. This is due, first of all, to their greater resistance (slower metabolism). Additionally, in order to reach insects, their larvae, pupae and eggs, lethal concentrations of the gas must penetrate into every hem and seam of, for example, clothing and bedding. Consequently, it also penetrates into every crack, crevice, and gap in a gassing facility. Warm-blooded animals, by contrast, are rapidly exposed to high concentrations of the gas, not only because of their size, but above all due to their continuous breathing through lungs.
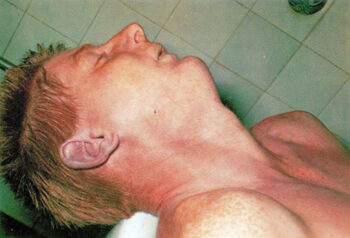
Most witnesses falsely described victims of Zyklon-B poisoning as black, blue, green or purple (e.g. Michał Kula, Filip Müller, Milton Buki, Pery Broad, Walter Petzold, Jan Wolny, Józef Weber, Aleksander Germański, Tadeusz Kurant, Wiesław Kielar, Ludwik Banach, Josef Klehr). Since a pinkish-red discoloration of the skin is not what people expect to see when confronted with victims of suffocation – be it by means of poison gas or simple oxygen deprivation – the sight of such pinkish-red corpses should have left a distinct impression in the memory of basically all those who claim to have witnessed it. Yet the rule is that almost all witnesses making statements about this followed the beaten path of a false cliché. This is just one piece of evidence that suggests that mass homicidal gassings with hydrogen cyanide never occurred.
A common German trivial name for hydrogen cyanide is “Blausäure”, meaning “blue acid.” The name is misleading for two reasons. First, this substance is not blue, but completely colorless, and second, it is only an acid when dissolved in water, but even then only to a very weak degree. The substance was given the name blue in the German-speaking world because hydrogen cyanide was first obtained in Germany through the decomposition of the blue pigment Iron Blue, a compound of oxidized iron (rust) and hydrogen cyanide (see the entry on Iron Blue). The name “blue acid” led some witnesses to claim that they had seen the poison gas as a blue haze, which is evidently untrue. (See for example the entry on Richard Böck.)
Lethal Concentration
Lethal doses of cyanide can be ingested orally, inhaled, or absorbed through the skin. Oral poisoning (for example with potassium cyanide, KCN) is very painful due to muscular convulsions (cramps) caused by cell suffocation. Inhalation poisoning induces unconsciousness faster, but can still cause painful convulsions, depending on the circumstances.
A dose of 1 mg cyanide per kg body weight is generally considered lethal for humans. Non-lethal doses of cyanide are quickly decomposed and excreted by the body.
Absorption through the skin is especially likely when the skin has become moist, for example, as a result of sweating. Thus, it is generally advised to avoid sweating during the handling of hydrogen cyanide. For poisoning through the skin, concentrations from 6,000 parts per million (0.6% by volume) constitute a health hazard, while 10,000 parts per million (1% by volume) can cause a lethal poisoning within just a few minutes.
Lethal concentrations of HCN given in today’s literature are based on experiments conducted with rabbits before World War One. More recent tests have shown that humans are less susceptible to HCN poisoning than small mammals such as rabbits (McNamara 1976). Furthermore, when considering the intention of killing all individuals exposed to HCN (such as in a large gas chamber with multiple victims), different standards must be used than those given in toxicological literature, which are not only based on inapplicable animal models, but moreover keep lethal threshold levels intentionally low to protect sensitive individuals. However, healthy and fit individuals can survive higher doses of toxins than weak or sick individuals.
Calculations and extrapolations from more-realistic animal models have resulted in a concentration, lethal within ten minutes for 100% of all individuals, of some 4,400 mg of hydrogen cyanide per m³ of air, or some 0.4%. This is close to the concentration that used to be applied during executions in U.S. gas chambers, where the average time until death was reportedly about 10 minutes, with extreme cases up to almost 20 minutes.
Hence, in order to kill all individuals in a hypothetical gas chamber within ten minutes, an average concentration during those ten minutes of some 0.4% would have to be applied. Faster execution times would require proportionally higher concentrations, with a time of five minutes requiring almost 1% of HCN in the air, which is a commonly used concentration during fumigations, also in disinfestation chambers, in order to kill pests such as fleas and lice. (See Rudolf 2023, esp. pp. 227-236.)
Chemical Traces
When coming in contact with material containing iron oxide (rust), hydrogen cyanide can form durable components under certain circumstances. These components are bluish in color. They can withstand decades of exposure to the elements, hence lend themselves as an indicator of previous exposure to hydrogen cyanide. See the entry on Iron Blue for more details on this.

You need to be a registered user, logged into your account, and your comment must comply with our Acceptable Use Policy, for your comment to get published. (Click here to log in or register.)